Abstract
Multiple myeloma (MM) is one of the many types of hematological malignancies, and many novel drugs, including histone deacetylase (HDAC) inhibitors such as panobinostat (pan-HDAC), romidepsin (HDAC1-3-specific), ACY-1215 and ACY-241 (HDAC6-specific) are currently undergoing preclinical and clinical evaluation.
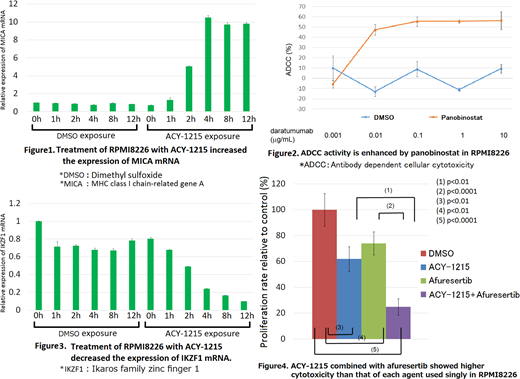
Daratumumab, an anti-CD38 monoclonal antibody, is a promising agent showing high activity in relapsed/refractory MM. However, some patients become resistant to daratumumab, partly due to the reduced CD38 expression in MM cells. It was previously shown that panobinostat increased CD38 expression in a time-dependent manner, particularly after 72 h (García et al). In our study, treatment with panobinostat for 24 h did not change the expression of CD38. Moreover, the expression of MHC class I chain-related gene A (MICA) was increased by all the examined HDAC inhibitors following treatment for 24 h (Figure 1). We established luciferase-expressing MM cell lines and examined antibody-dependent cell-mediated cytotoxicity (ADCC) in a luciferase assay. ADCC was enhanced after 24 h-treatment with the examined HDAC inhibitors (Figure 2). These results suggest that induction of MICA plays an important role in the enhancement of ADCC by HDAC inhibitors.
Furthermore, HDAC inhibitors decreased the expression of CD55 and CD59. Presumably, CD55 and CD59 inhibit complement activation and suppress complement-dependent cytotoxicity (CDC). We found the possibility of CDC enhancement of daratumumab by HDAC inhibitors in the luciferase assay.
CD47 in tumor cells inhibits phagocytosis. Blockade of interaction between CD47 in tumor cells and signal regulatory protein α (SIRP-α) in macrophages increases tumor cell phagocytosis in hematological malignancies. Moreover, the treatment of MM cells with HDAC inhibitors decreased CD47 expression. Thus, the possibility of enhancement of antibody-dependent cellular phagocytosis (ADCP) by daratumumab in these cells was suggested.
Ikaros family zinc finger 1 (IKZF1) is a transcription factor and pivotal target of immunomodulatory drugs (IMiDs). IMiDs mediate the binding of IKZF1 to cereblon complex and degrades IKZF1, thereby causing the loss of interferon regulatory factor 4 and MYC expression. A previous report showed that IKZF1 could suppress the transcription of MICA (Fionda et al). Here, we found that MM cells treated with HDAC inhibitors showed downregulated expression of IKZF1 mRNA (Figure 3). These results suggest the possibility that HDAC inhibitors increase MICA expression via inhibition of IKZF1, its negative regulator. IKZF1 could be a novel target of HDAC inhibitors, including panobinostat, romidepsin, ACY-1215 and ACY-241. Furthermore, it was presumed that IKZF1 is suppressed by HDAC inhibitors in a cereblon-independent manner.
Afuresertib is an oral AKT inhibitor, which has been clinically tested in patients with advanced hematological malignancies. Single-agent afuresertib showed a favorable safety profile and demonstrated clinical activity against patients with MM in phase I of a clinical trial (Spencer et al). In a proliferation assay, we found that afuresertib combined with HDAC inhibitors showed higher cytotoxicity than that of each agent used singly (Figure 4). The expression of cleaved caspase 3 and caspase 7 was induced more in MM cells treated with a combination of HDAC and AKT inhibitors than in those treated with only one agent. These results suggested that the combination of HDAC and AKT inhibitors strongly induces the apoptosis of MM cells. Moreover, the addition of AKT inhibitors to HDAC inhibitors caused a decrease in CD55, CD59 and CD47 expression.
The findings of the present study suggest the possibility that combination therapy with HDAC and AKT inhibitors could overcome drug resistance via multiple action mechanisms. Firstly, HDAC inhibitors could enhance ADCC owing to increased MICA expression, and these may be effective in patients with reduced CD38 expression during daratumumab therapy. Secondly, the possibility of IKZF1 decrease by HDAC inhibitors in a cereblon-independent manner was presumed. Thirdly, a combination of HDAC and AKT inhibitors induced strong apoptosis in MM cells, and it may enhance the CDC and ADCP of daratumumab. These data suggest that the addition of HDAC and AKT inhibitors to daratumumab could be an effective therapy for relapsed/refractory MM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal